Spinal Muscular Atrophy and Myostatin
$SRRK, $BHVN, $RHBBY all have upcoming readouts. Let's break it down
Highlights:
3 key catalysts in the next 6 months.
Biohaven 2H24 - phase 3 from taldefgrobep alfa in the Resilient trial
Roche Q125 - dose finding interim data in the phase 3 MANATEE trial with GYM329
Scholar Rock Q425 - Phase 3 Sapphire Trial with Apitegromab will read out
Myostatin inhibition failed in Duchenne Muscular Dystrophy showing how treating a neuromuscular disease is more than ‘give people steroids.’
Mice have higher levels of myostatin and human patients with neuromuscular disease also have reduced levels of myostatin so translation from preclinical models is difficult
Spinal Muscular Atrophy awareness is high. Patients have effective therapies to delay progression, but a major unmet need is muscle strength. SMN based therapies tend to plateau after 2-3 years of treatment.
Scholar Rock’s phase 2 data is messy because they’ve sliced the patient population in uncomfortable ways even if the drug shows some activity. The target Phase 3 population doesn’t make sense to me.
Furthermore, Roche’s GYM329 will likely have a long-term advantage with easier administration. The high price of incumbent therapies means any additional drug will face insurance scrutiny.
Quick Plug
I found these catalysts by using my catalyst tracker here: www.subradata.com/catalyst_data_display.
Check out my website at www.subradata.com which has a way to get updates on clinical trial protocol changes, a catalyst tracker, and consolidates information about a company: for example. Www.subradata.com/company/srrk. If you have any concerns, questions, or tips to make any part of the website better, contact me via DM, Email, telegraph, any other method of communication.
Also here’s the usual link a spreadsheet with a landscape overview: SMA Spreadsheet
Overview
Here’s ChatGPT’s explanation:
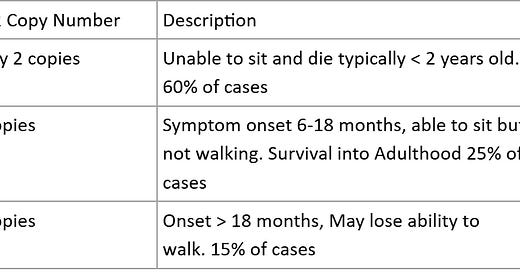
“Spinal Muscular Atrophy (SMA) is like a faulty electrical system in a house, where the wiring that connects the brain (control center) to the muscles (appliances) is damaged. The disease occurs because of a mutation in the SMN1 gene, which normally produces a protein essential for motor neurons. Without enough of this protein, the motor neurons (the "wires") begin to break down, leading to muscle weakness and loss of control over movement. The number of SMN2 gene copies, which act like backup generators, determines the severity of SMA. People with fewer SMN2 copies (1 or 2) have more severe forms, like SMA Type 1, which usually appears before six months of age and affects muscles needed for breathing and swallowing, much like appliances that can't even turn on. With more copies (3 or 4), people may develop milder forms like SMA Type 3, where symptoms appear later in childhood or adulthood, affecting walking and motor functions but allowing some appliances to run with reduced power. Each type of SMA reflects how much function the backup system can support before the motor neurons—and muscles—deteriorate.”
Pretty Good!
Spinal muscular atrophy (SMA) is a progressive disease caused by mutations in the Survival Motor neuron (SMN1) gene. Patients usually have proximal weakness, progressing distally leading to eventual disability. A dysfunctional SMN1 leads to neuronal death, but the SMN2 gene can compensate for issues with SMN1.
Current Therapies:
The three approved therapies—Spinraza, Evrysdi, and Zolgensma—all aim to increase SMN protein levels. Evrysdi is popular because it's oral, while Spinraza remains an option due to some patients having side effects from Evrysdi. Insurance won’t cover combination therapies, so any add-on treatment needs to bring something significant to the table. Evrysdi side effects : r/spinalmuscularatrophy (reddit.com). I highly suggest browsing the reddit if interested in knowing more.
Market Dynamics:
Evrysdi overtook Spinraza in Q1 2024 in sales, thanks to easier administration and better insurance coverage. But unmet needs remain: improving muscle strength, fatigue, and daily function are top priorities for patients. https://x.com/jordixiol/status/1783087979394646253
Early Diagnosis and treatment: Every state screens newborns for SMA starting this year. the change was made because of an emphasis on early treatment leading to better outcomes. 100% of States Now Screening Newborns for SMA - Cure SMA.
Data supports presymptomatic treatment with SMN2 therapies: Clinical Evidence Supporting Early Treatment Of Patients With Spinal Muscular Atrophy: Current Perspectives - PMC (nih.gov),
Early treatment is a lifeline for infants with SMA | Nature Medicine.
Current Unmet need:
SMN2 therapies were a revolution because a progressive, often fatal, disease was reversed but a plateau in treatment effecs and inability to restore full function leads to a high unmet need.
" Individuals with SMA and their families viewed gaining muscle strength (90%), achieving new motor function (73%), improving daily functioning (68%), reducing fatigue (53%), and improving swallowing (36%) and communication (21%) as their top priorities for future research and treatments." Cure-SMA.
SMN also plays a role in the skeletal muscle and Spinraza (Nusinersen), an intrathecal injection and does not affect the skeletal muscle. On the other hand, Evrysdi may have efficacy issues for some patients (source). Biogen tried addressing potential efficacy issues with high dose Spinraza but results seem disappointing. (source). It 'met the primary endpoint' but the primary endpoint compared it to sham control treatment, not low dose active therapy Standard dose Nusinersen shows ~20 pt difference in vs the 25-point difference in high dose Nusinersen (appendix, ENDEAR trial). Plus, Why not tell us about the data from both groups if it's positive?
We've established a few things
Evrysdi overtook Spinraza in Q1 2024 because it's oral and better covered by insurance.
Spinraza has a loyal fanbase but is fading.
Muscle improvements (not just survival) are the next big unmet need in SMA.
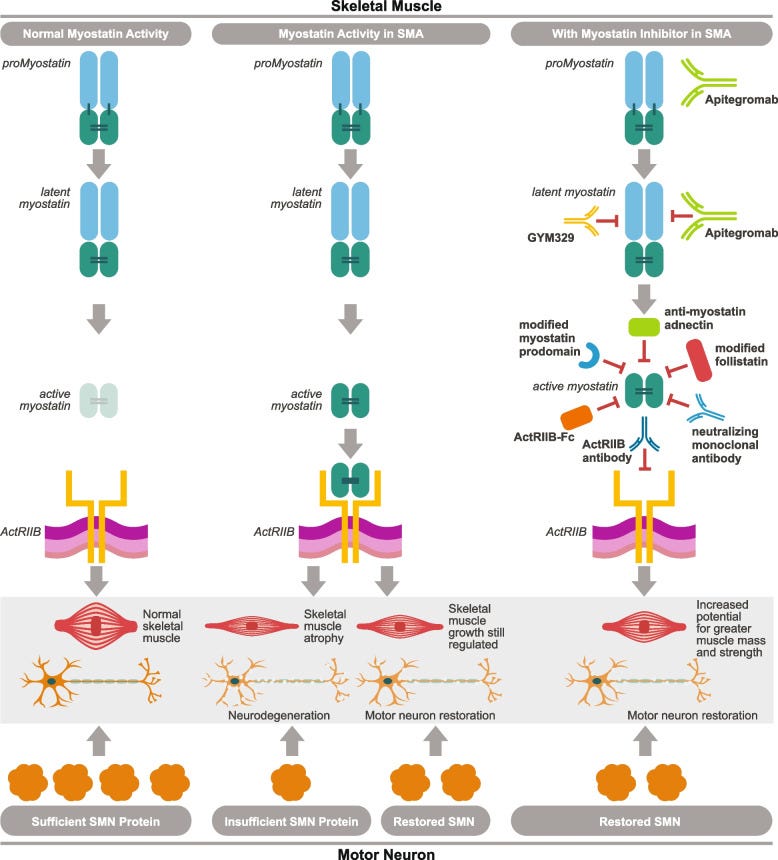
Myostatin inhibitors: A way to address muscle
The logic for myostatin inhibition is simple:
myostatin stops muscle growth
SMA is a mixed muscle/CNS disease
adding a drug which inhibits myostatin will improve SMA pathology by increasing skeletal muscle function.
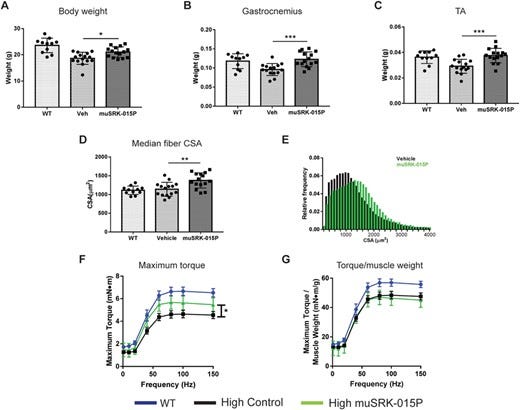
Preclinical data in mice do show additive benefits for combining SMN2 drugs and myostatin inhibitors for moderate to severe SMA mice (source, AAV- myostatin inhibitor plus ASO). All outcomes were generally positive including total muscle mass, neuronal function and survival. The best results were in the least severe mice. Other studies also show minimal effect in severe SMA mice reinforcing the potential for myostatin in less severe SMA phenotypes (Type 3, ambulatory) (source). The data also supports one explanation for prior failures in DMD: patients present with low myostatin levels so the drug can’t inhibit anything! (source)
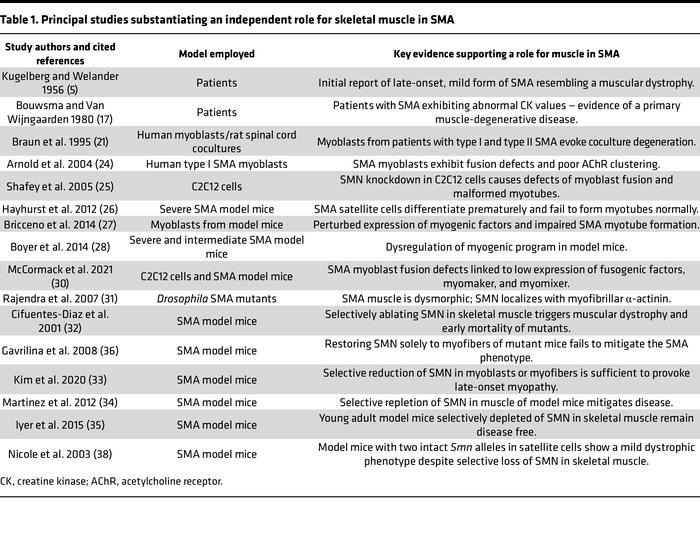
Preclinical data points support a pathological role of Muscle in SMA:
The pathology of SMA can be recapitulated simply by losing SMN in skeletal muscles. It suggests SMA is a combined CNS and muscular disease, correlated by the current unmet needs in improving motor function. The thesis is proven repeatedly in mice but yet to be substantiated by human trials.
Preclinical data from Scholar rock:
The preclinical data for scholar rock is messy and imperfect. Mice have higher circulating levels of myostatin (also why mice models don’t great for obesity, maybe need activin inhibition, topic for another day) yet the benefits accrued were marginal. It’s not bad, but it’s a far cry from a home run especially with the difficulties translating myostatin inhibition into the clinic.
Failure in DMD:
Myostatin (specifically taldefgrobep alfa) previously failed in Duchenne Muscular Dystrophy. The preclinical data supported a treatment effect, but the drugs failed to translate. I believe it’s due to 1) higher levels of myostatin in mice (humans have 10x lower levels) and 2) Even lower levels of myostatin in human disease patients (discussed below). The Failed Clinical Story of Myostatin Inhibitors against Duchenne Muscular Dystrophy
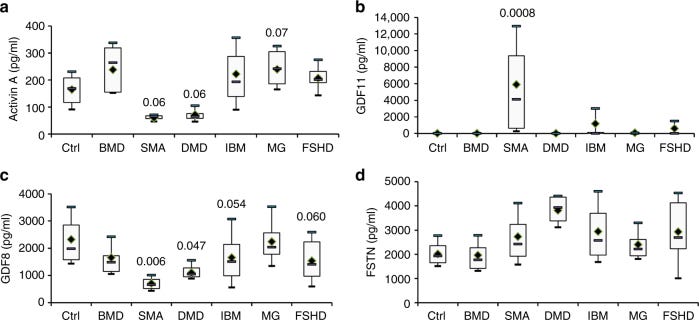
Human data on myostatin levels in SMA:
A few small studies assess the role of myostatin as a biomarker. A small Brazilian study observed minimally higher myostatin in patients on treatment, but notably lower myostatin in SMA patients. (source).
Another study assess myostatin levels as a biomarker for disease severity and found the following: Myostatin inversely correlated with severity, minimal correlation with weight, and minimal difference after SMN treatment.
Myostatin levels correlated with motor scores and outcomes, but the treatment itself doesn't seem to change the levels. The data is further supported by another assessment of myostatin as a biomarker.
My take on Data to date in preclinical models and circulating myostatin.
SMA is a combined muscle and CNS disease in mice. Adding myostatin inhibition to SMN based therapy is additive.
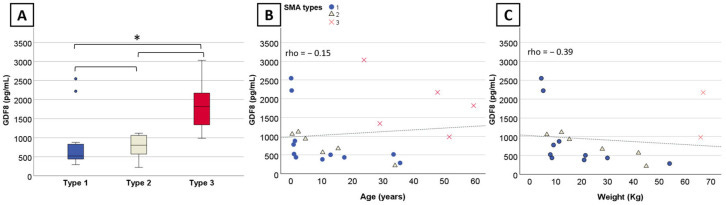
Myostatin levels are low in SMA patients as a marker for disease severity. More severe patients have lower myostatin levels. The ideal patient for a myostatin inhibitor is a type 3 patient with abundant myostatin
Most SMN therapies do not impact myostatin levels dramatically
Mice get more jacked on these drugs than humans.
Human Clinical trials:
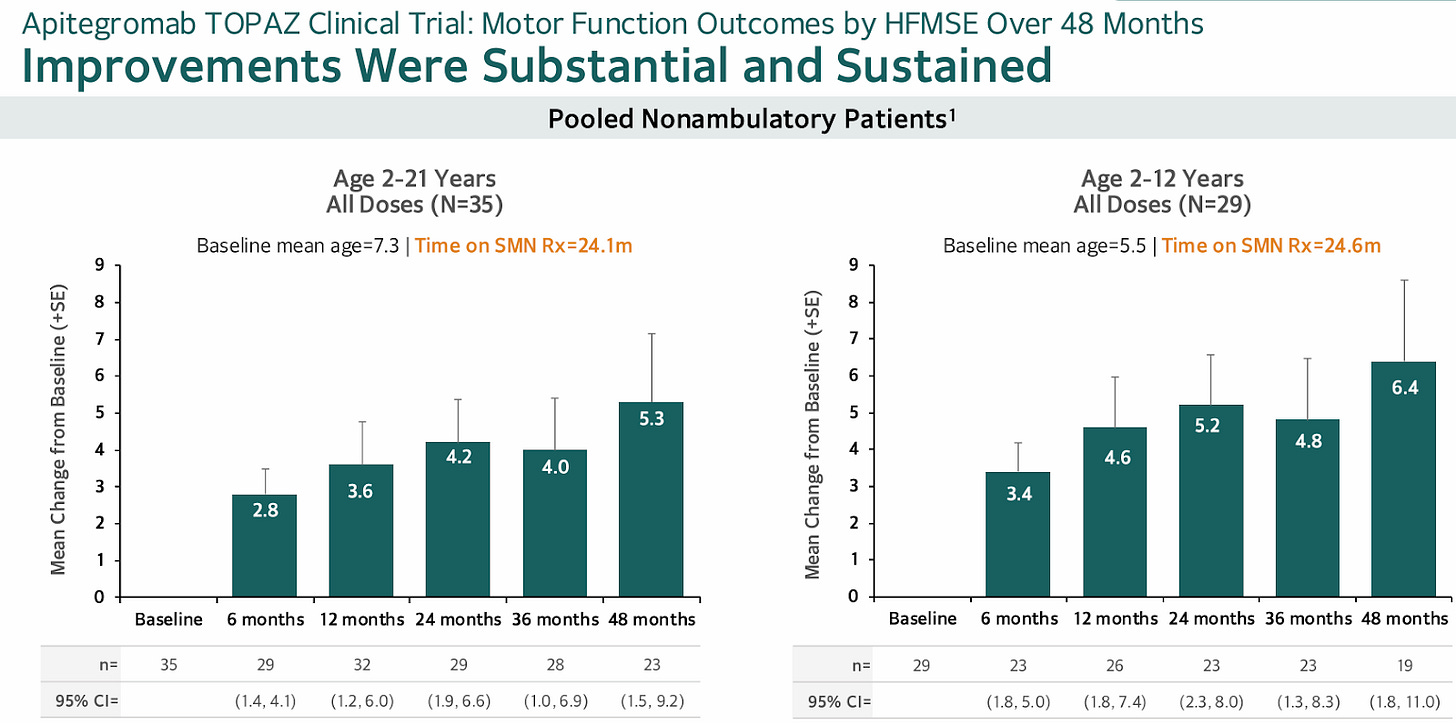
It's surprising to see only one, open label, phase 2 trial testing myostatin inhibition: TOPAZ. The data from Topaz, while small and open label, are somewhat encouraging. The trial tested 3 cohorts treated with apitegromab: ambulatory 5-21 year old patients, non-ambulatory 5-21 year olds, and non-ambulatory > 2 years old. Non ambulatory patients ages 2-12 had the greatest benefit on treatment.
Scholar Rock, slices and dices the data and presents long term follow-up in these patients showing a quick benefit and gradual increase over time.
Here's my take: The data doesn't make a whole lot of sense
One would think myostatin inhibition works best for ambulatory patients with higher myostatin levels to start. Here we see the opposite. More severe patients do better?
In addition, the analysis excludes patients who have scoliosis surgery during treatment: From Topaz
"The post hoc observation that showed an inverse relationship between 2 musculoskeletal complications, scoliosis and contracture, and change from baseline on the respective primary functional motor scales is notable. It suggests that these orthopedic limitations identify an unrecognized intermediate term marker of worsening and that they present a primary impediment to apitegromab action, are a marker of some other factor(s) limiting apitegromab response,"
If you have scoliosis, the response is worse yet they exclude these patients?
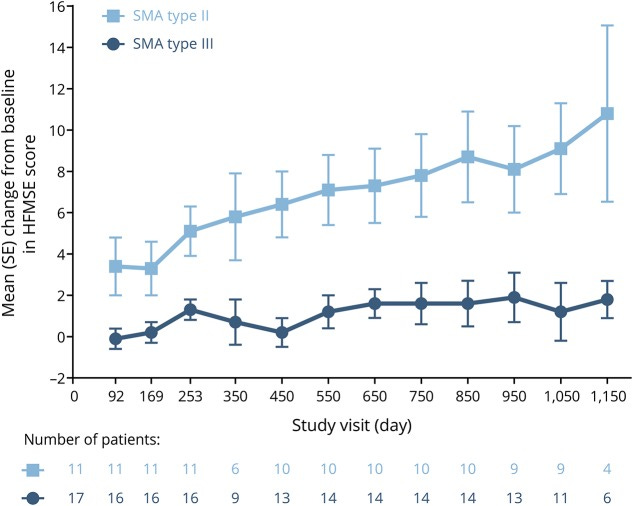
Long term HFMSE with Spinraza doesn’t plateau in younger, non ambulatory, patients. Nusinersen in later-onset spinal muscular atrophy - PMC (nih.gov).
Another consideration is how HFMSE changes as patients age. The gains in a younger cohort could simply be a function of growing older. The RULM, meant to assess strength more than gross function, isn't as positive on an initial jump.
On the positive side, the immediate jump in HFMSE, some supporting evidence of a higher dose cohort leading to faster gains, and substantial increase indicate some treatment effect
I think the trial is a 50/50 shot and I lean negative. A clinically meaningful difference is 3 points on the HFMSE and it's designed to hit that mark. It's a risk some might be willing to take.
Biohaven, Roche: two competitors hot on SRRK’s heels
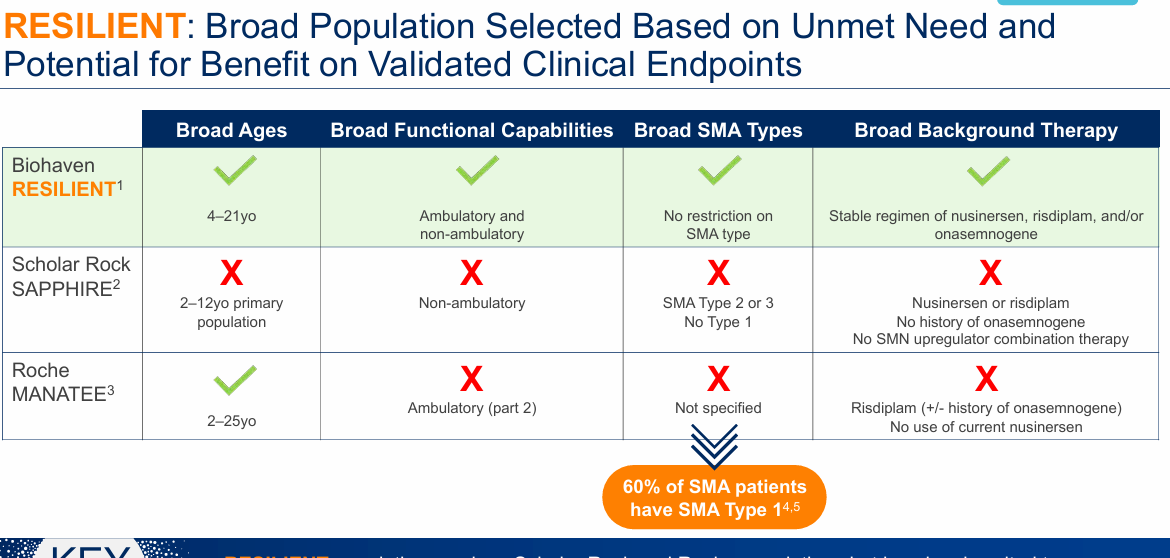
For Biohaven, they're taking the shotgun approach to their trial:
"try to hit the broad age range as well. As long as there's functional muscle, there's reason to believe that they could respond to a myostatin inhibitor like to talk about on top of the standard of care."
The primary outcome is different the MFM-32. I have the same concerns about myostatin inhibition as a mechanism for human SMA patients, but at least they include an older, less severe population in the trial. It feels like they’re running the trials based on vibes rather than solid proof of concept and prior clinical experience.
Roche's GYM329 will readout interim results in early 2025 (recently pushed back). GYM 329 is SQ IV Q4W with the same mechanism of action as Scholar Rock(target latent myostatin). Part 1 of their phase 3 trial will test the drug in non-ambulatory and ambulatory patients ages 2-10. Part 2 is ambulatory patients 2-25. I still have concerns on the mechanism of action but the preclinical data support Roche's approach. Myostatin should work in patients with the most muscle. Patients are only allowed to have background risdiplam as part of the trial which limits the market.
Thoughts on the stocks: I think you could play SRRK for a trade, but the long term investment thesis isn’t solid in my opinion.
My Bottom Line
Nusinersen demonstrated a strong benefit which plateaus after 2-3 years. The plateau is more pronounced in Type 3 patients.
The unmet needs to maintain muscle mass and function in SMA is high. Awareness of the condition is at all time high with newborn screening.
The Phase 2 TOPAZ trial shows some drug activity but the subgroup analysis makes no sense to me. Myostatin inhibition should work in patients with the highest myostatin levels which are ambulatory, older patients. They decided to run a trial in younger, non ambulatory patients. They've sliced and diced the data in uncomfortable ways.
HFMSE gains in TOPAZ (phase 2) the drug is active for some patients. The trial is well powered to hit stat sig. The bar for insurance coverage will be higher
Prior failures of myostatin in other muscular dystrophies can be attributed to 1) mice have higher levels of myostatin and 2) patients with DMD have low levels of myostatin. Seems like we can't just give people steroids to help with muscle disease. . Myostatin levels are low in SMA, lower in more severe patients, and no clear correlation between prior therapy and myostatin
Biohaven is taking a broad approach with no proof-of-concept data. Sounds fun.
Roche is targeting the right set of patients (in my opinion) but only allows background Evrysdi or Zolgensma.
Scholar rock's drug is IVQ4W and targets latent myostatin, Taldefgrobep alfa is SQ QW and targets myostatin directly (messier), GYM329 is SQ Q4W and targets latent myostatin. Do with that what you will.
DM or comment any thoughts you have.